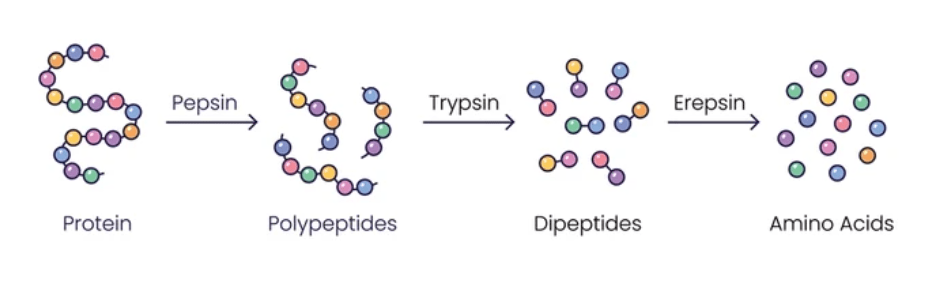
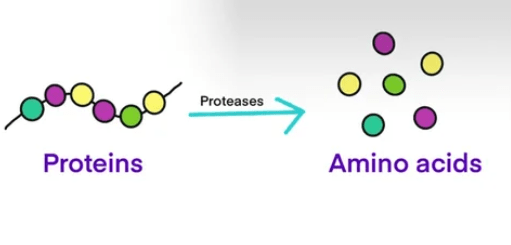
What are Proteins?
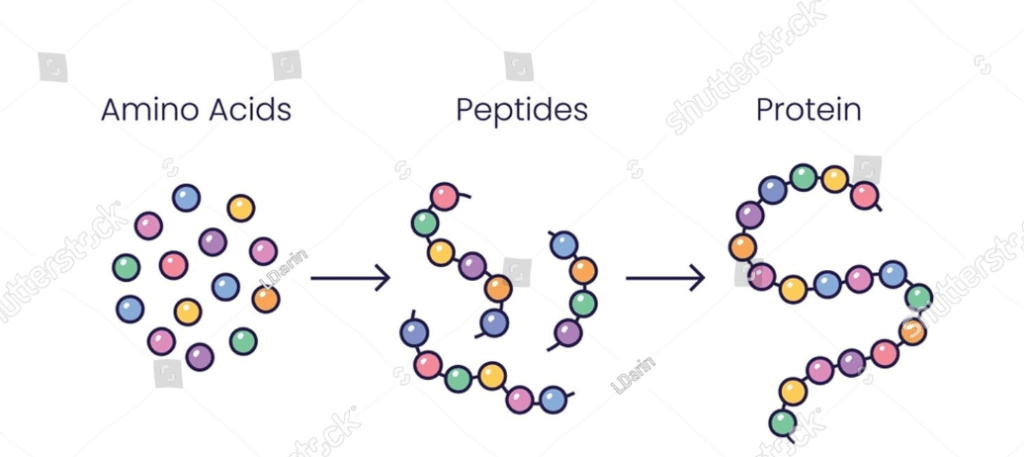
Proteins are large biomolecules made up of amino acid chains that are essential for the structure, function, and regulation of the body’s cells, tissues, and organs. They play diverse roles in biological processes, including enzymatic catalysis, structural support, transport of molecules, signaling, immune responses, and gene regulation. Proteins are composed of one or more polypeptide chains, each consisting of a linear sequence of amino acids linked together by peptide bonds. The specific sequence of amino acids in a protein determines its unique structure and function.
Peptide Linkages
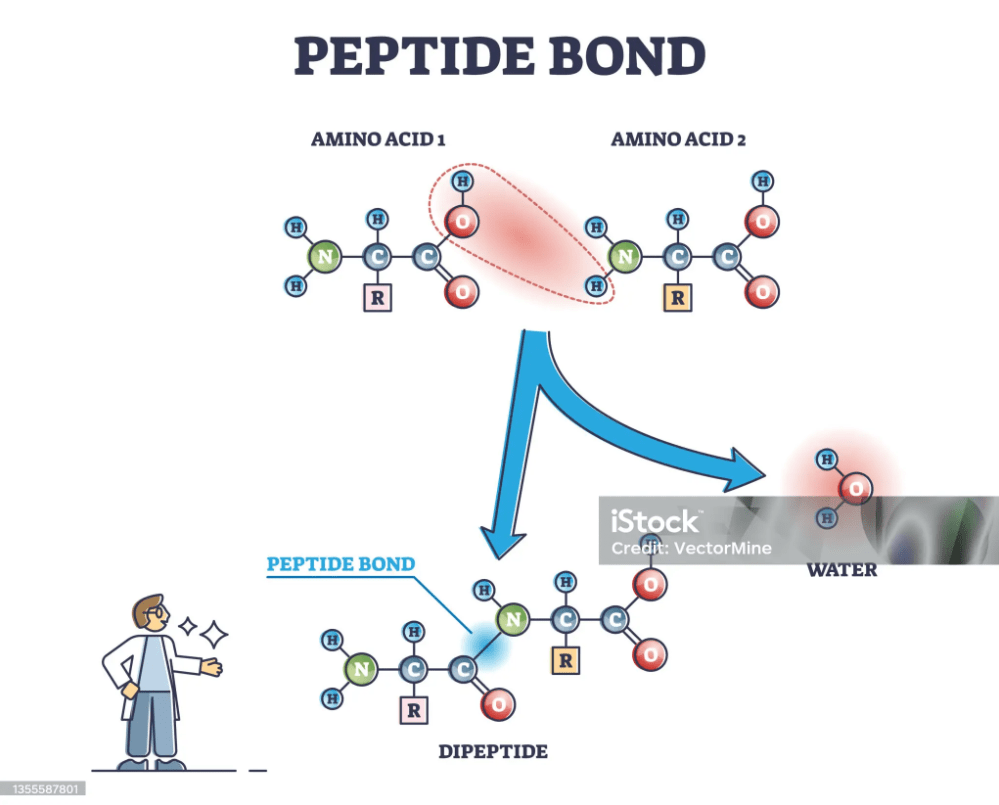
A peptide linkage, also known as a peptide bond, is a covalent chemical bond that links two consecutive amino acid monomers along a peptide or protein chain. This bond forms between the carboxyl group of one amino acid and the amino group of another, resulting in the release of a molecule of water (H₂O) — a type of dehydration synthesis reaction. The general chemical structure of a peptide bond is (-CO-NH-), and it plays a crucial role in the primary structure of proteins.
Here’s a step-by-step outline of how a peptide bond forms:
- Two Amino Acids: Start with two amino acids. Each amino acid has an amino group (-NH2) and a carboxyl group (-COOH).
- Dehydration Synthesis: The carboxyl group of the first amino acid reacts with the amino group of the second amino acid.
- Formation of the Peptide Bond:
- The carboxyl group of the first amino acid loses an -OH group.
- The amino group of the second amino acid loses a hydrogen atom (H).
- The -OH and H combine to form a molecule of water (H₂O).
- A new bond is formed between the carbon atom of the carboxyl group from the first amino acid and the nitrogen atom of the amino group from the second amino acid.
The result is a dipeptide with a peptide bond between the two amino acids:
Amino Acid_1 – CO – NH – Amino Acid_2 + H₂O
Characteristics of Peptide Bonds:
- Planarity: The peptide bond is rigid and planar due to the resonance between the carbonyl group and the nitrogen.
- Partial Double Bond Character: The bond has partial double-bond character because of resonance, which restricts rotation around the bond.
- Stability: Peptide bonds are generally stable under physiological conditions, but can be broken down by hydrolysis, often catalyzed by enzymes known as peptidases or proteases.
Importance of Peptide Bonds in Biology:
Peptide bonds are fundamental in forming the backbone of proteins, linking amino acids into polypeptide chains which then fold into specific three-dimensional structures essential for biological function. The sequence and nature of the amino acids in the polypeptide chain determine the protein’s structure and function.
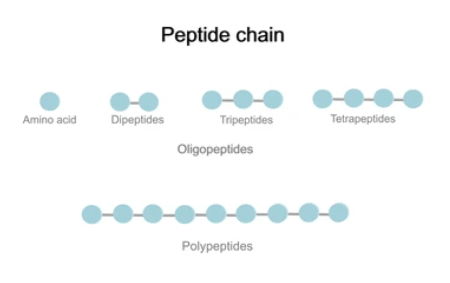
Types of Peptide Chains
Peptide chains are composed of amino acids linked together by peptide bonds. Depending on their composition and structure, peptide chains can be classified into different types.
Here are some common types of peptide chains:
1. Polypeptides
Definition: Polypeptides are linear chains of amino acids linked by peptide bonds. They typically contain more than 10 amino acid residues and can vary greatly in length, ranging from relatively small peptides to large proteins.
Characteristics:
- Formed by the polymerization of amino acids through peptide bond formation.
- Can contain various secondary structures such as alpha helices, beta sheets, or random coils.
- Play diverse roles in biological processes, including enzyme catalysis, structural support, and signaling.
2. Oligopeptides
Definition: Oligopeptides are short chains of amino acids containing fewer than 10 amino acid residues. They are intermediates between peptides and polypeptides, often serving as precursors to larger protein molecules.
Characteristics:
- Comprise a small number of amino acid residues, typically 2 to 9.
- Can exhibit secondary structures depending on their sequence and environment.
- Found in various biological contexts, including peptide hormones, neurotransmitters, and signaling molecules.
3. Dipeptides
Definition: Dipeptides are the simplest type of peptide chains consisting of only two amino acid residues linked by a single peptide bond.
Characteristics:
- Comprise two amino acids joined by a peptide bond.
- Lack the complexity of larger peptides and proteins.
- Serve as building blocks for larger peptides and proteins and can be generated by enzymatic hydrolysis of proteins.
4. Tripeptides
Definition: Tripeptides are peptide chains containing three amino acid residues linked together by two peptide bonds.
Characteristics:
- Comprise three amino acids joined by two peptide bonds.
- Can exhibit limited structural diversity and flexibility compared to longer peptides.
- Found in various biological molecules, including peptide hormones, antibiotics, and enzyme cofactors.
5. Polycyclic Peptides
Definition: Polycyclic peptides are cyclic peptide chains containing a closed ring structure formed by the covalent linkage of the peptide backbone.
Characteristics:
- Formed by the cyclization of linear peptide chains through the formation of peptide bonds between the N- and C-termini.
- Can exhibit enhanced stability and resistance to enzymatic degradation compared to linear peptides.
- Found in natural products, synthetic drugs, and bioactive peptides with diverse biological activities.
6. Multimeric Peptides
Definition: Multimeric peptides are peptide chains composed of multiple subunits or monomers, often assembled through non-covalent interactions or disulfide bonds.
Characteristics:
- Comprise multiple peptide subunits arranged in a specific spatial arrangement.
- Can exhibit cooperative interactions and complex biological functions not observed in monomeric peptides.
- Found in various protein complexes, such as enzymes, receptors, and structural proteins.
These are some common types of peptide chains found in biological systems, each with unique structural and functional properties. Understanding the characteristics and roles of different peptide chains is essential for elucidating their biological functions and applications in fields such as drug discovery, biotechnology, and chemical biology.
All the images are taken from the Shutterstock website.
N-Terminal and C-Terminal of a Peptide Chain
The N-terminal and C-terminal regions of proteins refer to specific ends of the polypeptide chain. These regions have distinct chemical and structural properties and play important roles in protein synthesis, structure, and function.
N-terminal (Amino Terminus)
- Definition: The N-terminal end of a protein is the starting point of the polypeptide chain. It corresponds to the free amino group (-NH2) of the first amino acid residue in the sequence.
- Characteristics:
- The N-terminal end is chemically reactive due to the presence of a free amino group.
- During protein synthesis, the N-terminal is the site of initiation, where the first amino acid is added to the growing polypeptide chain.
- The N-terminal region often contains targeting signals or signal peptides that direct proteins to specific cellular compartments or facilitate secretion.
- Functions:
- The N-terminal may contain important functional domains or motifs that mediate protein-protein interactions, enzyme activity, or binding to ligands.
- It can also be involved in post-translational modifications such as acetylation, phosphorylation, or myristoylation, which regulate protein stability, localization, or activity.
C-terminal (Carboxyl Terminus)
- Definition: The C-terminal end of a protein is the termination point of the polypeptide chain. It corresponds to the free carboxyl group (-COOH) of the last amino acid residue in the sequence.
- Characteristics:
- The C-terminal end is chemically reactive due to the presence of a free carboxyl group.
- During protein synthesis, the C-terminal is the site of termination, where the last amino acid is added to the polypeptide chain.
- The C-terminal region may contain sequences that influence protein folding, stability, or degradation.
- Functions:
- The C-terminal may contain important functional domains or motifs involved in protein-protein interactions, enzyme catalysis, or binding to substrates or cofactors.
- It can also serve as a recognition site for post-translational modifications such as proteolytic cleavage, ubiquitination, or glycosylation, which regulate protein function or turnover.
Importance
- The N-terminal and C-terminal regions are essential structural features of proteins that influence their folding, stability, localization, and interactions with other molecules.
- These regions can play critical roles in determining protein function, regulation, and signaling pathways.
- Understanding the properties and functions of the N-terminal and C-terminal ends is important for studying protein structure-function relationships, designing therapeutic agents, and engineering proteins for specific applications.