Amino acids are the building blocks of life, fundamental to the structure and function of proteins, which play crucial roles in virtually all biological processes. Let’s delve into what amino acids are, how they are classified, and their chemical properties.
What are Amino Acids?
Amino acids are organic compounds that contain both an amino group (-NH2) and a carboxyl group (-COOH). They are the monomers that polymerize to form proteins, which are essential macromolecules involved in nearly every cellular function, from catalyzing metabolic reactions to DNA replication and cell signaling.
Structure of Amino Acids
The general structure of an amino acid consists of:
- A central carbon atom (α-carbon).
- An amino group (-NH2).
- A carboxyl group (-COOH).
- A hydrogen atom (H).
- A distinctive side chain (R group) that varies among different amino acids and determines their unique properties.
Classifications of Amino Acids
Amino acids can be classified based on various criteria, including their chemical properties, nutritional requirements, metabolic pathways, and other biochemical characteristics. Here are some common bases of classification:
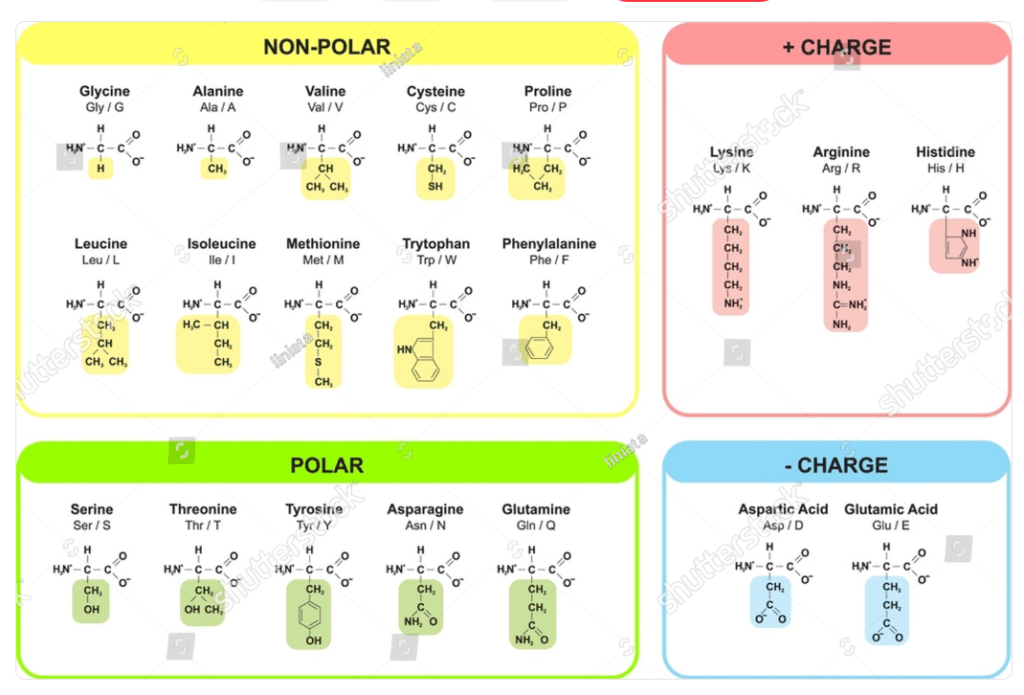
1. Based on Side Chain Properties
Amino acids can be classified based on the properties of their side chains (R groups). Each category reflects the chemical nature of the side chains, influencing the behavior and role of amino acids in proteins. Here’s a detailed explanation of each type:
Non-polar (Hydrophobic) Amino Acids
These amino acids have side chains that are non-polar and hydrophobic, meaning they tend to avoid water and are usually found in the interior of proteins. They play a key role in maintaining protein structure through hydrophobic interactions.
- Glycine (Gly): The simplest amino acid, with a single hydrogen atom as its side chain. It is small and flexible.
- Alanine (Ala): Has a methyl group (-CH3) as its side chain, making it small and hydrophobic.
- Valine (Val), Leucine (Leu), Isoleucine (Ile): These have branched alkyl side chains, making them highly hydrophobic.
- Methionine (Met): Contains a sulfur atom within a larger non-polar side chain.
- Phenylalanine (Phe): Has a benzyl group, contributing to its hydrophobic character.
- Tryptophan (Trp): Contains a bulky indole side chain, which is hydrophobic.
- Proline (Pro): Unique due to its cyclic structure that links back to the amino group, creating rigidity.
Polar (Uncharged) Amino Acids
These amino acids have side chains that are polar and can form hydrogen bonds with water and other polar molecules, but they do not carry a charge at physiological pH. They often participate in the active sites of enzymes or are involved in hydrogen bonding within proteins.
- Serine (Ser), Threonine (Thr): Contain hydroxyl groups (-OH) which are polar and can form hydrogen bonds.
- Cysteine (Cys): Contains a thiol group (-SH) that can form disulfide bonds, crucial for protein structure.
- Tyrosine (Tyr): Has a hydroxyl group attached to an aromatic ring, making it polar and capable of hydrogen bonding.
- Asparagine (Asn), Glutamine (Gln): Contain amide groups in their side chains, which are polar and can participate in hydrogen bonding.
Positively Charged (Basic) Amino Acids
These amino acids have side chains that are positively charged at physiological pH. They are hydrophilic and often participate in ionic interactions and hydrogen bonding.
- Lysine (Lys): Has a long aliphatic chain ending in an amino group, which is positively charged.
- Arginine (Arg): Contains a guanidinium group, making it very basic and positively charged.
- Histidine (His): Has an imidazole ring that can be positively charged, often found in enzyme active sites.
Negatively Charged (Acidic) Amino Acids
These amino acids have side chains that are negatively charged at physiological pH. They are hydrophilic and often involved in ionic interactions and hydrogen bonding.
- Aspartic Acid (Asp), Glutamic Acid (Glu): Contain carboxyl groups in their side chains, which are negatively charged.
Summary Table
| Category | Amino Acids | Characteristics |
|---|---|---|
| Non-polar (Hydrophobic) | Glycine (Gly), Alanine (Ala), Valine (Val), Leucine (Leu), Isoleucine (Ile), Methionine (Met), Phenylalanine (Phe), Tryptophan (Trp), Proline (Pro) | Hydrophobic side chains, typically found in the protein interior. |
| Polar (Uncharged) | Serine (Ser), Threonine (Thr), Cysteine (Cys), Tyrosine (Tyr), Asparagine (Asn), Glutamine (Gln) | Polar side chains, capable of hydrogen bonding, usually on protein surfaces or active sites. |
| Positively Charged (Basic) | Lysine (Lys), Arginine (Arg), Histidine (His) | Positively charged side chains, hydrophilic, involved in ionic interactions. |
| Negatively Charged (Acidic) | Aspartic Acid (Asp), Glutamic Acid (Glu) | Negatively charged side chains, hydrophilic, involved in ionic interactions. |
2. Based on Nutritional Requirements
Amino acids are essential for building proteins, but not all amino acids can be synthesized by the human body. Some amino acids must be obtained from the diet, as the body cannot produce them in sufficient quantities. These are called essential amino acids. Here’s an explanation based on nutritional requirements:
Essential Amino Acids
These are amino acids that the body cannot synthesize on its own, so they must be obtained from the diet. They are essential for protein synthesis and various physiological functions. A deficiency in any essential amino acid can lead to impaired protein synthesis, resulting in health problems.
- Histidine (His): Required for the growth and repair of tissues, as well as the production of histamine, a neurotransmitter involved in immune response and digestion.
- Isoleucine (Ile): Important for muscle metabolism, energy regulation, and hemoglobin synthesis.
- Leucine (Leu): Plays a crucial role in protein synthesis, muscle repair, and growth. It also regulates blood sugar levels and provides energy to muscles during exercise.
- Lysine (Lys): Essential for protein synthesis, collagen formation, calcium absorption, and hormone production.
- Methionine (Met): Required for protein synthesis, methylation reactions, and the production of important molecules like creatine and glutathione.
- Phenylalanine (Phe): Necessary for protein synthesis and the production of neurotransmitters such as dopamine, adrenaline, and noradrenaline.
- Threonine (Thr): Important for protein synthesis, immune function, and the maintenance of gut health.
- Tryptophan (Trp): Precursor to serotonin, a neurotransmitter involved in mood regulation and sleep. It also plays a role in protein synthesis and immune function.
- Valine (Val): Essential for muscle metabolism, tissue repair, and the maintenance of nitrogen balance in the body.
Non-essential Amino Acids
These are amino acids that the body can synthesize on its own, so they do not need to be obtained from the diet. However, they are still important for various physiological processes and can become conditionally essential in certain situations, such as illness or stress.
- Alanine (Ala)
- Asparagine (Asn)
- Aspartic Acid (Asp)
- Glutamic Acid (Glu)
Conditionally Essential Amino Acids
These are amino acids that are typically non-essential but become essential under certain conditions, such as illness, stress, or metabolic disorders. In these situations, the body’s demand for these amino acids exceeds its ability to produce them.
- Arginine (Arg): Important for wound healing, immune function, and the production of nitric oxide, a molecule that regulates blood flow.
- Cysteine (Cys): Required for the synthesis of glutathione, a powerful antioxidant, and for the formation of disulfide bonds in proteins.
- Glutamine (Gln): Essential for immune function, gut health, and the maintenance of nitrogen balance in the body.
- Glycine (Gly): Involved in the synthesis of proteins, DNA, and neurotransmitters. It also plays a role in detoxification and the production of creatine.
- Proline (Pro): Important for the structure and stability of proteins, especially collagen, the main structural protein in connective tissue.
- Serine (Ser): Required for the synthesis of proteins, nucleotides, and phospholipids. It also plays a role in neurotransmitter metabolism and immune function.
- Tyrosine (Tyr): Precursor to neurotransmitters like dopamine, adrenaline, and noradrenaline. It also plays a role in protein synthesis and the production of thyroid hormones.
| Category | Amino Acids |
|---|---|
| Essential | Histidine (His), Isoleucine (Ile), Leucine (Leu), Lysine (Lys), Methionine (Met), Phenylalanine (Phe), Threonine (Thr), Tryptophan (Trp), Valine (Val) |
| Non-essential | Alanine (Ala), Asparagine (Asn), Aspartic Acid (Asp), Glutamic Acid (Glu) |
| Conditionally Essential | Arginine (Arg), Cysteine (Cys), Glutamine (Gln), Glycine (Gly), Proline (Pro), Serine (Ser), Tyrosine (Tyr) |
3. Based on Metabolic Pathways
Amino acids can be classified based on their metabolic fate, which refers to how they are utilized within the body for energy production, protein synthesis, or other biochemical processes. Here’s an explanation based on metabolic fate:
Glucogenic Amino Acids
These amino acids can be converted into intermediates of the gluconeogenesis pathway, ultimately leading to the production of glucose. They serve as precursors for glucose synthesis during periods of fasting, low carbohydrate intake, or intense exercise when glucose levels need to be maintained.
- Examples: Alanine, Arginine, Asparagine, Aspartic Acid, Cysteine, Glutamic Acid, Glutamine, Glycine, Histidine, Methionine, Proline, Serine, Threonine, Valine.
Ketogenic Amino Acids
These amino acids can be converted into ketone bodies, such as acetoacetate and β-hydroxybutyrate, which can be used as alternative fuel sources, particularly by the brain, during prolonged fasting or low carbohydrate intake.
- Examples: Leucine, Lysine.
Glucogenic and Ketogenic Amino Acids
These amino acids can be converted into both glucose precursors and ketone bodies, providing flexibility in their metabolic fate depending on the body’s energy needs and nutritional status.
- Examples: Isoleucine, Phenylalanine, Threonine, Tryptophan, Tyrosine.
Non-Glucogenic and Non-Ketogenic Amino Acids
These amino acids are not directly involved in glucose or ketone body production and may have specific roles in protein synthesis, neurotransmitter synthesis, or other metabolic pathways.
- Examples: Glycine, Alanine (can be both glucogenic and ketogenic), Cysteine, Proline.
| Category | Amino Acids |
|---|---|
| Glucogenic | Alanine (Ala), Arginine (Arg), Asparagine (Asn), Aspartic Acid (Asp), Cysteine (Cys), Glutamic Acid (Glu), Glutamine (Gln), Glycine (Gly), Histidine (His), Methionine (Met), Proline (Pro), Serine (Ser), Threonine (Thr), Valine (Val) |
| Ketogenic | Leucine (Leu), Lysine (Lys) |
| Both Glucogenic and Ketogenic | Isoleucine (Ile), Phenylalanine (Phe), Threonine (Thr), Tryptophan (Trp), Tyrosine (Tyr) |
4. Based on Structure of Side Chains
Amino acids can be classified based on the structure of their side chains (R groups), which significantly influences their chemical properties and functions within proteins. Here’s an explanation of each type based on the structure of side chains:
Aliphatic Amino Acids
These amino acids have side chains that consist of aliphatic hydrocarbon groups, which are straight or branched chains of carbon atoms.
- Examples: Glycine, Alanine, Valine, Leucine, Isoleucine.
Characteristics: Aliphatic amino acids are generally non-polar and hydrophobic, making them suitable for protein cores and membrane-spanning regions.
Hydroxylic Amino Acids
These amino acids contain hydroxyl (-OH) groups in their side chains.
- Examples: Serine, Threonine.
Characteristics: The presence of hydroxyl groups makes these amino acids polar and capable of forming hydrogen bonds, influencing protein structure and function.
Sulfur-Containing Amino Acids
These amino acids contain sulfur atoms in their side chains, typically in the form of thiol (-SH) groups.
- Examples: Cysteine, Methionine.
Characteristics: Cysteine plays a crucial role in forming disulfide bonds, stabilizing protein structures. Methionine is involved in protein synthesis and various metabolic processes.
Aromatic Amino Acids
These amino acids feature aromatic rings in their side chains, which are composed of six carbon atoms arranged in a cyclic structure.
- Examples: Phenylalanine, Tyrosine, Tryptophan.
Characteristics: Aromatic amino acids are often involved in protein-ligand interactions, enzyme catalysis, and signal transduction due to their unique chemical properties.
Cyclic Amino Acids
These amino acids have side chains that form cyclic structures.
- Examples: Proline.
Characteristics: Proline’s cyclic side chain restricts its conformational flexibility, making it a structural determinant in protein folding and stability.
Acidic and Amides Amino Acids
These amino acids have side chains containing carboxyl (-COOH) or amide (-CONH2) groups.
- Examples: Aspartic Acid, Glutamic Acid, Asparagine, Glutamine.
Characteristics: Aspartic acid and glutamic acid are negatively charged at physiological pH, contributing to protein charge distribution and ionic interactions. Asparagine and glutamine are involved in hydrogen bonding and protein synthesis.
Basic Amino Acids
These amino acids feature side chains containing amino (-NH2) groups or other basic functional groups.
- Examples: Lysine, Arginine, Histidine.
Characteristics: Basic amino acids are positively charged at physiological pH, allowing them to participate in ionic interactions and hydrogen bonding, influencing protein stability and function.
Understanding the structural diversity of amino acids provides insights into their roles in protein structure, function, and interaction within biological systems. This classification helps elucidate the relationships between amino acid properties and their contributions to the complexity and functionality of proteins.
| Category | Amino Acids |
|---|---|
| Aliphatic | Glycine (Gly), Alanine (Ala), Valine (Val), Leucine (Leu), Isoleucine (Ile) |
| Hydroxylic | Serine (Ser), Threonine (Thr) |
| Sulfur-Containing | Cysteine (Cys), Methionine (Met) |
| Aromatic | Phenylalanine (Phe), Tyrosine (Tyr), Tryptophan (Trp) |
| Cyclic | Proline (Pro) |
| Acidic and Amides | Aspartic Acid (Asp), Glutamic Acid (Glu), Asparagine (Asn), Glutamine (Gln) |
| Basic | Lysine (Lys), Arginine (Arg), Histidine (His) |
5. Based on Functional Group
Amino acids can also be classified based on the functional groups present in their side chains, which greatly influence their chemical properties and roles in biological systems. Here’s an explanation of each type based on functional groups:
Alkyl Group Amino Acids
These amino acids have side chains composed primarily of alkyl groups, which are chains of carbon and hydrogen atoms.
- Examples: Glycine, Alanine, Valine, Leucine, Isoleucine.
Characteristics: Alkyl groups are non-polar and hydrophobic, making these amino acids typically found in the interior of proteins where they contribute to protein stability.
Hydroxyl Group Amino Acids
These amino acids contain hydroxyl (-OH) groups in their side chains.
- Examples: Serine, Threonine, Tyrosine.
Characteristics: Hydroxyl groups make these amino acids polar and capable of forming hydrogen bonds. They play roles in protein structure, enzyme catalysis, and signal transduction.
Thiol Group Amino Acid
This amino acid has a thiol (-SH) group in its side chain.
- Example: Cysteine.
Characteristics: Cysteine’s thiol group can form disulfide bonds with other cysteine residues, contributing to protein folding, stability, and the formation of protein structures like hair and nails.
Aromatic Group Amino Acids
These amino acids feature aromatic rings in their side chains.
- Examples: Phenylalanine, Tyrosine, Tryptophan.
Characteristics: Aromatic rings confer unique chemical properties, allowing these amino acids to participate in π-π stacking interactions, hydrophobic interactions, and various protein-ligand interactions.
Amide Group Amino Acids
These amino acids contain amide (-CONH2) groups in their side chains.
- Examples: Asparagine, Glutamine.
Characteristics: Amide groups can participate in hydrogen bonding, contributing to protein stability and structure. Asparagine and glutamine are also involved in protein synthesis and nitrogen metabolism.
Carboxyl Group Amino Acids
These amino acids have carboxyl (-COOH) groups in their side chains.
- Examples: Aspartic Acid, Glutamic Acid.
Characteristics: Carboxyl groups are negatively charged at physiological pH, making these amino acids acidic. They participate in ionic interactions, protein-ligand binding, and enzymatic catalysis.
Amino Group Amino Acids
These amino acids feature amino (-NH2) groups in their side chains.
- Examples: Lysine, Arginine, Histidine.
Characteristics: Amino groups are positively charged at physiological pH, making these amino acids basic. They play roles in ionic interactions, pH buffering, and enzymatic catalysis.
Understanding the functional groups present in amino acids is essential for comprehending their biochemical roles, interactions, and contributions to protein structure and function. This classification helps elucidate the diverse chemical properties of amino acids and their importance in biological systems.
| Category | Amino Acids |
|---|---|
| Alkyl Group | Glycine (Gly), Alanine (Ala), Valine (Val), Leucine (Leu), Isoleucine (Ile) |
| Hydroxyl Group | Serine (Ser), Threonine (Thr) |
| Thiol Group | Cysteine (Cys) |
| Phenyl Group | Phenylalanine (Phe) |
| Benzyl Group | Tyrosine (Tyr) |
| Indole Group | Tryptophan (Trp) |
| Imidazole Group | Histidine (His) |
| Amide Group | Asparagine (Asn), Glutamine (Gln) |
| Carboxyl Group | Aspartic Acid (Asp), Glutamic Acid (Glu) |
| Amino Group | Lysine (Lys), Arginine (Arg) |
This table summarizes the various ways amino acids can be classified based on their chemical properties, nutritional requirements, metabolic pathways, structural characteristics, and functional groups. Each classification highlights different aspects of amino acid diversity and functionality.
Click here to practice the structures of all amino acids
Chemical Properties of Amino Acids
Amino acids exhibit various chemical properties that stem from their unique molecular structures, including their side chains (R groups) and functional groups. Here are some key chemical properties of amino acids:
1. Acid-Base Properties:
- Amino acids contain both acidic (carboxyl group, -COOH) and basic (amino group, -NH2) functional groups.
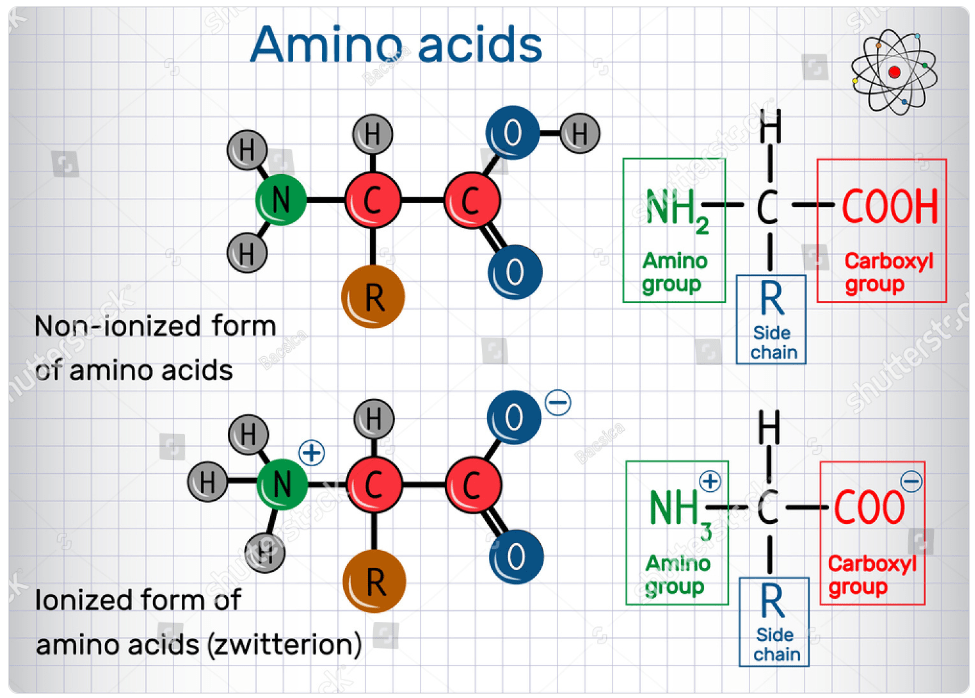
- At physiological pH (~7.4), the carboxyl group is deprotonated (-COO-) and the amino group is protonated (-NH3+), resulting in the zwitterionic form of the amino acid.
- Amino acids can act as both acids and bases, allowing them to participate in acid-base reactions.
2. Ionization:
- Amino acids can exist in different ionization states depending on the pH of their environment.
- At low pH, amino acids are mostly protonated and positively charged.
- At high pH, amino acids are mostly deprotonated and negatively charged.
- The isoelectric point (pI) is the pH at which an amino acid has no net charge and exists as a zwitterion.
3. Hydrophobicity and Hydrophilicity:
- Amino acids exhibit varying degrees of hydrophobicity and hydrophilicity depending on the nature of their side chains.
- Non-polar (hydrophobic) amino acids have side chains that interact poorly with water.
- Polar (hydrophilic) amino acids have side chains that can form hydrogen bonds with water molecules.
4. Hydrogen Bonding:
- Amino acids with polar or charged side chains can form hydrogen bonds with other molecules, including water, other amino acids, and small molecules.
- Hydrogen bonding contributes to protein structure, stability, and interactions with ligands or substrates.
5. Chirality:
- Except for glycine, all amino acids are chiral molecules, meaning they have two enantiomers (L and D forms).
- In proteins, only the L-forms of amino acids are typically found, with L-alpha amino acids being the primary building blocks.
6. Redox Reactions:
- Amino acids containing sulfur, such as cysteine and methionine, can participate in redox reactions.
- Cysteine can form disulfide bonds (-S-S-) through oxidation, contributing to protein folding and stability.
7. Reactivity:
- Amino acids can undergo various chemical reactions, including condensation, hydrolysis, oxidation, and reduction.
- The reactivity of amino acids is influenced by their functional groups and side chain properties.
8. Zwitter Ion Formation:
A zwitterion, also known as a dipolar ion or inner salt, is a molecule that contains both positively and negatively charged functional groups, but overall has a net neutral charge. In the context of amino acids, the zwitterionic form is the predominant state of amino acids in aqueous solutions, especially at physiological pH.
The formation of a zwitterion in amino acids occurs due to the presence of both an amino group (-NH2) and a carboxyl group (-COOH) in the same molecule. At physiological pH, which is around 7.4, the amino group acts as a base and accepts a proton (H⁺), becoming positively charged (NH₃⁺), while the carboxyl group acts as an acid and donates a proton, becoming negatively charged (COO⁻). This results in the formation of a zwitterion, where the positively charged amino group and the negatively charged carboxyl group cancel each other out, leaving the molecule with a net neutral charge.
The zwitterionic form of amino acids is important for their solubility in water and for their role in biological systems. It allows amino acids to form stable structures such as proteins and peptides, as well as to interact with other molecules in solution through various electrostatic interactions, hydrogen bonds, and hydrophobic interactions. Additionally, the zwitterionic form of amino acids plays a crucial role in the buffering capacity of biological systems, helping to maintain the pH balance within cells and tissues.
Understanding the chemical properties of amino acids is essential for comprehending their roles in protein structure, function, and interactions within biological systems. These properties govern amino acid behavior in physiological processes, enzyme catalysis, signaling pathways, and many other biochemical reactions.