The Michaelis-Menten equation describes the rate of enzymatic reactions by relating reaction rate to substrate concentration. Here’s a step-by-step derivation of the equation:
1. Enzyme-Substrate Complex Formation
Consider a simple enzymatic reaction where an enzyme ( E ) binds to a substrate ( S ) to form an enzyme-substrate complex ( ES ), which then breaks down to release a product ( P ) and regenerate the enzyme ( E ):

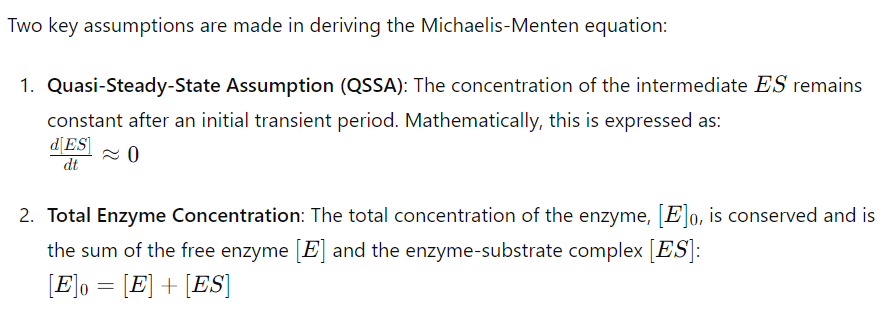
2. Assumptions
3. Formation and Breakdown of ( ES )
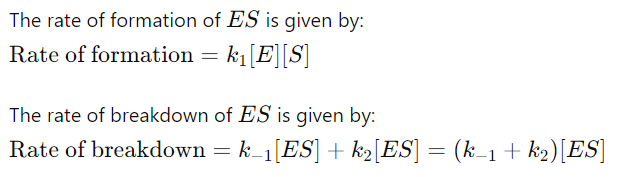
4. Applying the QSSA
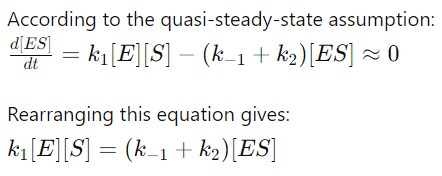
5. Expressing [E] in Terms of [E]0
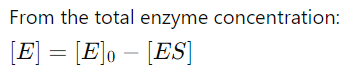
6. Substituting [E] into the QSSA Equation
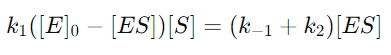
7. Solving for [ES]
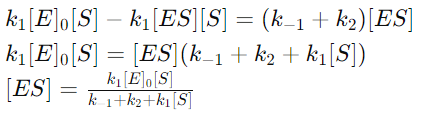
8. Introducing the Michaelis Constant (KM)
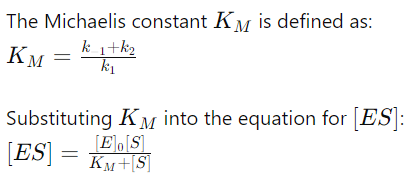
9. Rate of Product Formation
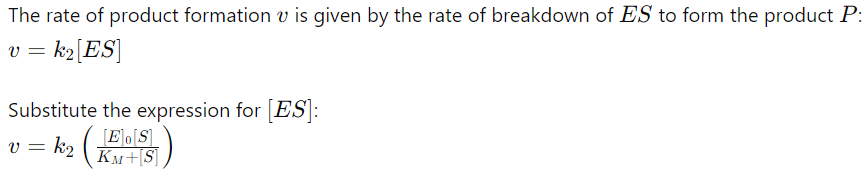
10. Maximum Rate (Vmax)
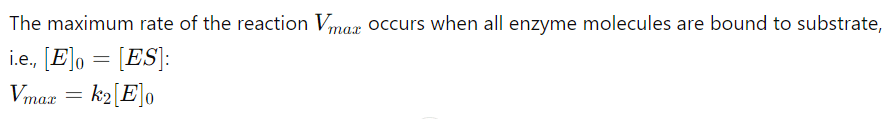
11. Final Michaelis-Menten Equation

Summary
